30 Apr Nitazoxanide And Colon Cancer
Colon cancer is the world’s third most lethal malignancy. In recent years, there has been substantial improvement in the treatment of this chronic condition. Many studies have found that different mediators have a role in practically every stage of colon carcinogenesis, including initiation, promotion, progression, and metastasis.
The emergence of gene mutations, such as genetic abnormalities associated to the wingless (Wnt)/-catenin signaling, is regarded to be the key driving factor in the early stages of colon cancer. The latter route is an unique signaling system that regulates colon cancer gene expression, migration, proliferation, and differentiation. -catenin, a crucial intermediary in Wnt signaling, is located in adherent junctions, the cytoplasm, and the nucleus; it regulates various biological interactions in these cellular compartments. A high amount of nuclear -catenin has been related to a poor prognosis in colon cancer patients, as well as greater vulnerability to cancer recurrence and a worse survival rate. Among the molecular regulators of Wnt signaling is glycogen synthase kinase-3 (GSK-3), a multifunctional serine/threonine kinase that regulates a number of cellular processes. Overexpression of Wnt or -catenin proteins has been shown to increase the expression of proliferating cell nuclear antigen (PCNA).
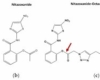
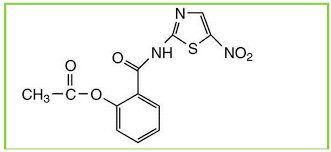
Romark Laboratories introduced nitazoxanide [2-(acetyloxy)-N-(5-nitro-2-thiazolyl)benzamide] as an antiparasite drug in 2002. Some studies have found that nitazoxanide has anti-cancer activity. Nitazoxanide demonstrated potent anti-cancer efficacy in a variety of cancer cells and tumor types. Nitazoxanide’s anti-cancer efficacy is explained by a variety of mechanisms, including autophagy and anti-cytokine activity. Nitazoxanide’s pharmacokinetic features include oral absorption and hydrolysis to its active metabolite, tizoxanide, which conjugates with glucuronide. Nitazoxanide is generally well tolerated, with no significant side effects in humans.
It is critical to identify key chemicals and signaling pathways in colon cancer and develop innovative treatments to target them. Wnt signaling has lately been proposed as a therapeutic target for colon cancer. The current work aimed to assess nitazoxanide’s cytotoxic efficacy in an in vitro colon cancer cell line and in vivo chemically generated colon cancer. The scope of this work was also expanded to investigate the effect of nitazoxanide on Wnt/-catenin signaling and tumor apoptosis.
Results
Nitazoxanide is being tested for its cytotoxic effect against HCT-116 colon cancer cells and FHC. The MTT findings showed that nitazoxanide had high cytotoxic activity against HCT-116, with an IC50 value of 11.07 M compared to the value observed with 5-flurouracil (5-FU, IC50 = 11.36 M), indicating that the compound was more cytotoxic than the 5-FU (Figure 1). It was also safe against FHC, with a rather high IC50 value of 48.4 M. As a result, the activity and selectivity of nitazoxanide in its action were concluded. As a result, flow cytometric analyses and gene expression levels were used to assess its apoptotic activity.
http://www.mhsvbrstudy.com/2020/01/08/what-are-mebendazoles/
Investigating Apoptosis Indicators
Cell-Cycle Analysis with Annexin/Propidium Iodide (PI) Staining
For 48 hours, HCT-116 cancer cells were treated with nitazoxanide (IC50 = 11.07 M). Using cell cycle analyses, we looked into its apoptotic activities. Nitazoxanide greatly boosted apoptotic cell death by a factor of 15.86. (28.72 percent versus 1.81 percent for the control). It increased early, intermediate, and late apoptotic cell death by 4.88, 15.26, and 8.58 percent, respectively. Furthermore, DNA flow cytometry was used to examine the cell cycle kinetics in HCT-116 cancer cells treated with nitazoxanide. Nitazoxanide substantially enhanced cell population in the G2/M cell phase (23.99 percent versus 8.08 percent in the control test) and cell population at the Pre-G1 cell phase (23.99 percent versus 8.08 percent in the control test) (28.72 percent compared to 1.81 percent in the control test). However, nitazoxanide had a non-significant effect on cell population in both stages S (39.67% drop compared to 49.31 percent for control) and G0/G1 (36.34 percent reduction compared to 42.61 percent for control). As a result, we may infer that nitazoxanide induced the arrest of the pre-G1 and G2/M cell cycles and slowed the development of HCT-116 cancer cells.
The real-time polymerase chain reaction (RT-PCR) experiments were performed on HCT-116 cells treated with nitazoxanide (IC50 = 11.07 M) for 48 hours. The mRNA expression of pro-apoptotic proteins (P53 and BAX), caspases (-3, -8, and -9), and the anti-apoptotic protein (BCL-2) was evaluated in HCT-116 cells. Nitazoxanide considerably increased the mRNA expression of P53 and BCL-2-associated X protein (4.09-fold) (BAX, 6.96-fold). Nitazoxanide also enhanced the mRNA levels of caspases 3 (8.49-fold), 8 (3.06-fold), and 9 (5.90-fold). Nitazoxanide, on the other hand, dramatically decreased BCL-2 mRNA expression (0.28-fold).
It is known that nitazoxanide was initially developed as an antiprotozoal medication and was licensed for the treatment of Cryptosporidium parvum and Giardia lamblia infections of the gut. Studies have also shown that nitazoxanide has a broad spectrum of activity against viruses, bacteria, parasites, and malignancies; however, the mechanism that nitazoxanide targets in human cells was previously unknown.
In the current investigation, nitazoxanide was investigated for its cytotoxic effect in two colon cancer models (in vitro and in vivo), as well as several mechanistic methods. In vitro, nitazoxanide was evaluated for its effect on apoptotic genes such as BAX, P53, caspase, and BCL-2, while in vivo, it was investigated for its effect on Wnt/-catenin/GSK-3 proteins. A molecular docking investigation validated the latter.
String databases, we believe, extract curated data from the following sources: systematic co-expression analysis, detection of shared selective signals across genomes, automated text-mining of scientific literature, and computational transfer of interaction knowledge between organisms based on gene ortholog. As a result, STRING analysis was performed to establish that the selection of our gene’s expression in HCT116 works with several other genes that may be involved in the whole process.
In the current in vitro investigation, a 48-h incubation period with the IC50 of nitazoxanide boosted the proportion of apoptotic cells in a colon cancer cell line. Furthermore, colon cancer cells expressed more mRNA for pro-apoptosis proteins such P53, BAX, and caspases (-3, -8, and -9), but less mRNA for BCL-2. The STRING bioinformatics database was used to illustrate the relationship between these genes. These findings were consistent with the network analysis performed with STRING. Apoptosis is a type of planned cell death. Extrinsic variables regulate p53 during apoptosis. The active p53 protein binds to a specific region in the DNA, prompting transcriptional activation of numerous genes implicated in apoptosis, such as the BCL-2 protein family. Furthermore, BAX alters the anti-apoptotic activity of the BCL-2 protein, resulting in the release of cystolic mitochondrial cytochrome c from human colon cancer cells. Additional intrinsic activators of the BCL-2 protein family regulate cytochrome c release.
According to the current findings, nitazoxanide has been shown to reduce tumor development by inhibiting c-Myc and triggering apoptosis in breast cancer xenografts in mice. Furthermore, nitazoxanide’s anti-cancer effects have already been investigated and verified in human colon cancer cell lines and animal models. Nitazoxanide inhibits important metabolic and pro-death signals such as autophagy, causing protein response, autophagy, anti-cytokine activities, and c-Myc suppression. A non-oncologic study found that nitazoxanide had a direct inhibitory impact on IL-6 production in both in vitro and in vivo mouse models. However, the mechanism by which nitazoxanide inhibited IL-6 synthesis is unknown.
Wnt/-catenin/GSK-3 proteins were shown to be increased in in vivo chemically induced colon cancer in mice. Wnt signaling is regarded as one of the most conserved pathways, with numerous key regulatory functions such as tissue homeostasis and biological processes. Wnt signaling pathways are classified as non-canonical -catenin-independent or canonical -catenin-dependent. In health, the latter plays balanced roles in physiologic processes and pathologic aspects of the adult intestine, such as preserving the crypt stem cell compartments. It promotes the development of colon cancer when induced by mutation. In colon cancer, abnormal Wnt/-catenin signaling causes chromosomal instability, according to the findings. Downregulation of the DICKKOPF-1 gene, a Wnt antagonist in colon cancer, provided evidence.
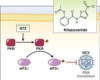
Nitazoxanide caused a dose-dependent suppression of Wnt/-catenin/GSK-3 protein synthesis in an in vivo colon cancer investigation. These decreases were followed by a shift in the synthesis of PCNA antigen, a -catenin downstream target. Overall, our findings support prior findings that nitazoxanide has an inhibitory effect on colon cancer growth via inhibiting -catenin.
In the current investigation, nitazoxanide inhibited the synthesis of Wnt and -catenin protein in the colon. In a cell culture-based screening, nitazoxanide stimulated the 5′ AMP-activated protein kinase (AMPK) signaling and downregulated the mechanistic target of rapamycin (mTOR) and Wnt signaling; the authors stated that this effect happened at therapeutically feasible dosages. The most likely mechanism is that nitazoxanide induces increased citrullination, which then stabilizes peptidyl arginine deiminase type-2, causing -catenin destruction.
Furthermore, as indicated by the Western blotting analysis, nitazoxanide inhibits GSK-3 expression. GSK-3 has previously been shown to have various impacts on cancer cells. GSK-3 negotiates the nuclear factor- (NF-B) route, and so plays an important role in cell survival, particularly in colon cancer, where coactivation for both the Wnt/-catenin and the NF-B pathways occurs due to ubiquitin system instability. Several studies found that treating colon cancer cell lines with different doses of GSK-3 inhibitors lowered cell viability and triggered apoptotic machinery in a dose-dependent manner.
http://www.mhsvbrstudy.com/2020/01/15/anthelmintics-explained/